
Many LifeDNA articles frequently reference Genome-Wide Association Studies (GWAS). But what exactly is GWAS, and why is it a recurrent topic in genetic research discussions? Furthermore, why do genetic testing companies, including LifeDNA, often cite these studies? This article delves into these questions, offering a clear perspective on the importance and relevance of GWAS in the field of genetics.
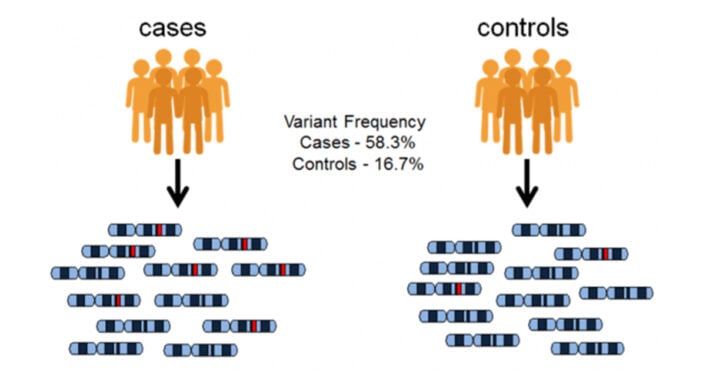
GWAS are well-controlled studies designed to find links between specific genetic variations and certain traits or diseases in a population. The ultimate aim is to understand diseases and traits at the genetic level, which could lead to preventing, treating, or managing them more effectively.
However, finding a genetic link does not immediately explain which gene is involved or how it causes the observed effects on the trait or disease. So, while GWAS can point out exciting connections, more research is needed to fully understand the underlying biology.
You may also like: 31 Frequently Asked Questions About Genetic Testing
Identifying genetic variants is the foundational goal of GWAS. Unraveling these variants allows researchers to pinpoint the precise changes in DNA sequences associated with specific traits or predispositions to diseases.
While humans share over 99% of their DNA sequences, alterations in the DNA sequence make one’s genome unique; these genetic variants can be a single nucleotide change or span large genome regions. The most common type of genetic variant is a single nucleotide polymorphism (SNP), where there is an alteration in a single base pair in the DNA sequence.
Age-Related Macular Degeneration (AMD)
One of the early successes of GWAS was the identification of several genetic variants linked to AMD, a leading cause of vision loss in older adults. Specifically, a variant in the complement factor H (CFH) gene was associated with a higher risk of developing the disease.
In one of the most comprehensive GWAS for AMD conducted in 2016, researchers analyzed DNA samples from 16,144 patients and 17,832 controls. The research pinpointed 52 distinct AMD-associated SNPs situated at 34 unique genomic locations. The genes are primarily involved in three key biological pathways: the complement cascade within the innate immune system, HDL transport, and the organization and assembly of the extracellular matrix. These pathways play a crucial role in the development and progression of AMD.
Type 2 Diabetes
GWAS and subsequent meta-analyses have helped pinpoint over 56 susceptibility sites for type 2 diabetes. These loci account for approximately 10% of the disease risk. An example is a variant in the TCF7L2 gene, associated with an increased risk of this form of diabetes.
Schizophrenia
Schizophrenia has a heritability of 60-80%. In recent years, GWAS on schizophrenia has identified numerous common susceptibility loci. These findings solidify the hypothesis of a significant polygenic influence, suggesting that many minor genetic effects together affect the disease onset. A GWAS published in April last year involving as many as 76,755 individuals diagnosed with schizophrenia and 243,649 control subjects, revealed associations with common genetic variants at 287 distinct locations on the genome.
One notable example is the variant in the MHC (major histocompatibility complex) region, which showed a strong association with this disorder.
Height
While genetics and environmental factors influence height, GWAS has identified several genetic variants associated with stature. A 2018 GWAS study on around 250,000 European participants identified approximately 700 SNPs linked to height.
By combining data with another GWAS on height and BMI from about 450,000 participants from the UK Biobank, also of European descent, the total sample size approached around 700,000 individuals. Specifically, researchers discovered 3,290 SNPs associated with height. Of these, 1,185 SNPs were in regions not pinpointed in prior GWAS. For instance, variants in the HMGA2 gene tend to influence height differences in individuals.
The largest GWAS ever on height was published in October 2022. This study, encompassing nearly 5.4 million subjects, uncovered 12,000 genetic variants associated with height, highlighting the very polygenic nature of this trait.
Breast Cancer
Research through GWAS has effectively pinpointed numerous genetic loci linked to the risk of breast cancer. These discoveries account for up to 18% of the heritability, underscoring that breast cancer is multifaceted with polygenic influences.
One such variant is in the FGFR2 gene. Women who carry this variant have a slightly higher risk of developing breast cancer than those who do not.
Lactose Intolerance
A variant in the LCT gene (rs4988235) is commonly associated with lactose intolerance in adults. Individuals with specific versions of this gene are less able to produce lactase as they grow older, which can lead to lactose intolerance.
You may also like: How Do Genes Influence Your Risk for Lactose Intolerance?
Fatty Acid Desaturase (FADS) Gene Cluster
Variants in the FADS1 and FADS2 genes are associated with the metabolism of omega-3 and omega-6 polyunsaturated fatty acids. These genetic differences can influence how people metabolize and utilize these essential fatty acids from their diet.
Varicose Veins
A 2019 GWAS, including nearly 10,000 cases and 300,000 controls, identified 30 genetic locations strongly linked with varicose veins. The most notable associations were found in the intron region of CASZ1 (rs1112165), previously implicated in blood pressure, and in the 16q24 region, where the PIEZO1 gene is located.
You may also like: How Do Genes Influence Your Risk for Varicose Veins?
Bitter Taste Perception
An SNP in the TAS2R38 gene, specifically rs713598, influences the ability to taste a specific bitter compound called phenylthiocarbamide (PTC). This genetic variation can affect individual dietary preferences and potential nutrient intake.
Chronotype (Morningness/Eveningness)
Several genetic variants influence whether you are a morning or evening person. For instance, a variant in the PER2 gene, known to be a part of the circadian clock mechanism, has been linked to evening preference.
You may also like: How Do Genes Influence Your Chronotype?
Sleep Disorders
Variants in the HLA-DQB1 gene are associated with narcolepsy, a sleep disorder characterized by excessive daytime sleepiness and other abnormal REM sleep manifestations. Furthermore, a 2010 association study discovered that the short allele of the 5-HTTLPR was significantly more common in individuals with insomnia compared to the control group (47.1% vs. 39.9%).
You may also like: How Do Genes Influence Your Risk for Insomnia?
Identifying common variations in DNA sequence deepens our understanding of the genetic underpinnings of traits and diseases and has practical implications. Knowing your genetic makeup and potential risk markers can lead to personalized medical approaches, better preventive strategies, and more targeted therapeutic interventions.
As detailed in the previous section, complex conditions, such as AMD, diabetes, and many psychiatric disorders, are polygenic and multifactorial, resulting from genetic and environmental factors interplay. They do not follow the straightforward inheritance patterns observed in single-gene (Mendelian) diseases, making them more challenging to study and understand. GWAS has proven instrumental in unraveling the genetic architecture of these complex diseases.
Multigenic Influences
Unlike Mendelian disorders, where one mutation in one gene leads to disease, complex conditions and traits often involve variations in multiple genes each with a small contribution to the overall risk. GWAS can scan the entire genome and pinpoint these numerous genetic variants, even if each has a tiny effect size.
Environmental Interactions
The risk of developing a complex condition depends on how genetic variations interact with environmental factors. For example, a person might have genetic variations associated with a higher risk of type 2 diabetes. However, if they maintain a healthy weight and diet, they may never develop the disease. GWAS can identify these genetic susceptibilities and pinpoint populations benefitting from specific preventive measures.
Gene-Environment-Wide Interaction Studies (GEWIS)
GEWIS is an advanced approach building on GWAS. GEWIS seeks to identify genes linked to diseases and how these genes interact with specific environmental factors. This holistic view can provide a more comprehensive understanding of traits and disease etiology.
Uncovering Biological Pathways
Identifying genetic variants associated with various conditions can reveal underlying biological pathways. For instance, GWAS findings have shed light on previously unknown pathways in diseases like rheumatoid arthritis and macular degeneration. This also paves the way for the development of new therapeutic targets.
Phenotypic Heterogeneity
Many complex diseases exhibit phenotypic heterogeneity, meaning different patients with the same illness might show diverse symptoms. GWAS can help identify genetic variations responsible for these differences. This enables clinicians to predict disease progression and outcome in a better way.
The field of medicine where this aspect of GWAS has produced tremendous results is psychiatric disorder research. Especially in psychiatry, GWAS has shown that mental illnesses and related complex characteristics often arise from the combined effects of many genes rather than just one or a few. This is called polygenicity.
Endophenotypes
Sometimes, the direct phenotypic outcome (like the actual condition or trait) might be hard to link to specific genetic variants. However, underlying traits or endophenotypes can be more directly tied to genetic factors. Endophenotypes are traits or characteristics that aren’t observable but can be measured. Blood pressure and cholesterol levels are examples of endophenotypes. GWAS on endophenotypes can help dissect the genetic components leading to the broader disease phenotype.
GWAS has transformed the landscape of risk prediction in healthcare. By identifying genetic variants associated with diseases, GWAS provides a foundation for understanding the genetic component of risk. This has led to the development of polygenic risk scores to ascertain the intensity of the risk.
Polygenic Risk Scores (PRS)
By aggregating the effects of multiple SNPs, researchers can calculate an individual’s cumulative genetic risk, known as a polygenic risk score. PRS can quantify an individual’s genetic predisposition to various diseases, from heart disease to certain types of cancer.
GWAS has provided an invaluable lens through which the public health community can understand disease patterns, risks, and susceptibilities. The insights from GWAS can have profound implications for public health policies and strategies. Here’s an exploration of how GWAS influences public health:
Targeted Health Interventions
GWAS uncovers genetic variants associated with diseases by allowing the design of tailored interventions. Populations with a high prevalence of specific genetic markers can be the focus of specialized prevention and treatment strategies and campaigns.
Informing Screening Programs
Knowledge of genetic susceptibilities can help design more efficient population-based screening programs. For instance, if a particular genetic variant linked to breast cancer is prevalent in a certain population, more rigorous and frequent mammography screening might be recommended.
Disease Surveillance and Monitoring
Public health surveillance systems can incorporate GWAS data to monitor trends in genetic susceptibilities over time. This could be essential for tracking emerging health threats or understanding the evolution of chronic diseases.
Educational Campaigns
With the information derived from GWAS, health authorities can devise educational campaigns that address the genetic aspects of diseases, helping the general public understand their risks and the importance of genetic testing.
Policy Formulation
Governments and health agencies can utilize GWAS insights to form policies related to healthcare funding, research priorities, and healthcare infrastructure development. This can help ensure the allocation of resources where they can have the most significant impact.
Collaborative Research
The vast datasets generated by GWAS can be shared among researchers globally, fostering collaboration. This collective effort can lead to faster discoveries, benefiting public health on a broader scale.
Ethical Considerations and Guidelines
As GWAS uncovers more about human genetics, it brings ethical considerations concerning genetic information privacy, discrimination, and informed consent to the forefront. This necessitates the development of robust ethical guidelines, ensuring that genetic information is used responsibly in the public health domain.
Precision medicine aims to tailor treatments to individual patients based on their genetic makeup. GWAS plays a crucial role in identifying the genetic variations that might determine how patients respond to treatments. The application of GWAS in personalized medicine is a vast topic, and we will discuss it in a future article.
In the context of GWAS, heritability (often denoted as H2) refers to the proportion of the variance in a trait attributed to genetic factors. Here is an example: If a trait (like height) has an H2 of 0.8, then 80% of the population’s height variability can be traced back to genetic differences.
However, GWAS often uncovers a mystery called “missing heritability.” Even after finding many associated genes, the cumulative effect of these genes often does not explain the entire heritability estimated for a trait. There’s a gap between the heritability based on familial patterns and the heritability we can pin down with GWAS. Understanding and solving this mystery is one of the ongoing challenges in the field.
While GWAS has revolutionized our understanding of the genetic basis of many conditions and diseases, they’re not without limitations. The sheer volume of data generated requires sophisticated tools and methodologies for analysis. Furthermore, the specific genetic variations identified only signify an association, not causation.
However, as technology evolves and our understanding deepens, GWAS will continue to be at the forefront of genetic research, potentially unveiling mysteries of our DNA that we have yet to imagine.
*Understanding your genetics can offer valuable insights into your well-being, but it is not deterministic. Your traits can be influenced by the complex interplay involving nature, lifestyle, family history, and others.
Our reports have not been evaluated by the Food and Drug Administration. The contents on our website and our reports are for informational purposes only, and are not intended to diagnose any medical condition, replace the advice of a healthcare professional, or provide any medical advice, diagnosis, or treatment. Consult with a healthcare professional before making any major lifestyle changes or if you have any other concerns about your results. The testimonials featured may have used more than one LifeDNA or LifeDNA vendors’ product or reports.


