Age-Related Macular Degeneration (AMD)
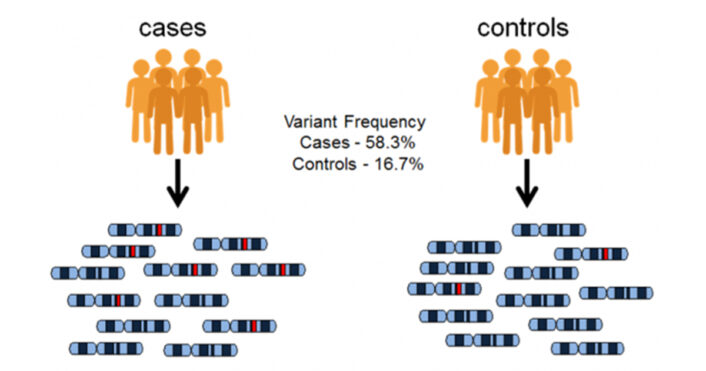
One of the early successes of GWAS was the identification of several genetic variants linked to AMD, a leading cause of vision loss in older adults. Specifically, a variant in the complement factor H (CFH) gene was associated with a higher risk of developing the disease.
In one of the most comprehensive GWAS for AMD conducted in 2016, researchers analyzed DNA samples from 16,144 patients and 17,832 controls. The research pinpointed 52 distinct AMD-associated SNPs situated at 34 unique genomic locations. The genes are primarily involved in three key biological pathways: the complement cascade within the innate immune system, HDL transport, and the organization and assembly of the extracellular matrix. These pathways play a crucial role in the development and progression of AMD.
Type 2 Diabetes
GWAS and subsequent meta-analyses have helped pinpoint over 56 susceptibility sites for type 2 diabetes. These loci account for approximately 10% of the disease risk. An example is a variant in the TCF7L2 gene, associated with an increased risk of this form of diabetes.
Schizophrenia
Schizophrenia has a heritability of 60-80%. In recent years, GWAS on schizophrenia has identified numerous common susceptibility loci. These findings solidify the hypothesis of a significant polygenic influence, suggesting that many minor genetic effects together affect the disease onset. A GWAS published in April last year involving as many as 76,755 individuals diagnosed with schizophrenia and 243,649 control subjects, revealed associations with common genetic variants at 287 distinct locations on the genome.
One notable example is the variant in the MHC (major histocompatibility complex) region, which showed a strong association with this disorder.
Height
While genetics and environmental factors influence height, GWAS has identified several genetic variants associated with stature. A 2018 GWAS study on around 250,000 European participants identified approximately 700 SNPs linked to height.
By combining data with another GWAS on height and BMI from about 450,000 participants from the UK Biobank, also of European descent, the total sample size approached around 700,000 individuals. Specifically, researchers discovered 3,290 SNPs associated with height. Of these, 1,185 SNPs were in regions not pinpointed in prior GWAS. For instance, variants in the HMGA2 gene tend to influence height differences in individuals.
The largest GWAS ever on height was published in October 2022. This study, encompassing nearly 5.4 million subjects, uncovered 12,000 genetic variants associated with height, highlighting the very polygenic nature of this trait.
Breast Cancer
Research through GWAS has effectively pinpointed numerous genetic loci linked to the risk of breast cancer. These discoveries account for up to 18% of the heritability, underscoring that breast cancer is multifaceted with polygenic influences.
One such variant is in the FGFR2 gene. Women who carry this variant have a slightly higher risk of developing breast cancer than those who do not.